The healthcare sector is facing cost pressures and increasing quality awareness
High requirements, increasing quality awareness, changing legal requirements and growing cost pressures in the healthcare sector are placing huge demands on manufacturers of medical devices. We offer businesses in the healthcare sector professional support in designing their business processes and in quality and change management. In the areas of serialisation and UDI in particular, rantronik is at your side as an expert consultant.
Meeting requirements and service provision in medical engineering
Quality is always at the heart of all of our projects, but especially where one of our key areas of expertise, medical engineering, is concerned. rantronik provides support in the field of quality management throughout the entire process. We advise you on preparing and reviewing SOPs and implementing GMP guidelines, CE certifications and CAPA management. We work together with you to restructure QM systems on the basis of new standards or legislation. We are happy to act as your point of contact for commissioning and approving processing equipment, in the context of business process, supplier and change management and technical due diligence checks. Working on the basis of a goal-oriented plan of action agreed with you, we ensure that the required results are obtained and we achieve quantifiable, sustainable success for your business.

UDI ‒ Unique Device Identification
Safety, traceability and transparency by means of unambiguous identification and entry in GUDID and EUDAMED databases
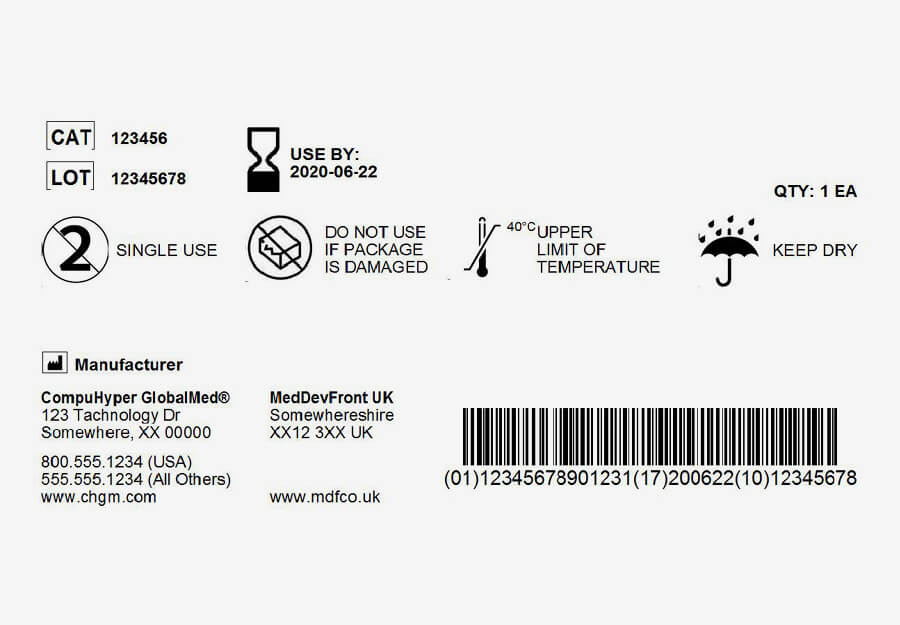
UDI standard on labelling medical devices
Under the 2013 FDA final rule, companies are required to unambiguously label and register pharmaceutical products and medical devices. This is for traceability purposes and to protect against counterfeit products, thereby improving patient safety and care. A machine-readable code and plain text label must be applied to every medical device. The European Database on Medical Devices (EUDAMED) and the Global UDI Database (GUDID) store information about individual products and can be accessed at any time.
Implementation of the UDI standard for medical products and pharmaceuticals companies
Class II and III medical devices exported to the USA are already subject to the UDI standard. Labelling must be in place for class I medical devices by September 2020. A UDI system is gradually being introduced in Europe as well, starting in May 2017, in the context of the new Medical Devices Regulation (MDR). The introduction of the UDI standard comprises more than just additional printing. First of all, the codes, encrypted in accordance with ISO standards, must be obtained from accredited agencies and contracting authorities. The codes are then applied to the products, whereby the printing or laser technology used must be adapted to the packing material in question. This requires investment in new hardware and software and integration of the necessary elements in existing plant and control systems. The quality and permanence of the 2D code must satisfy the official requirements and continuous quality management, including safety evaluations, must take place. Finally, comprehensive data management is necessary and must ensure that up to 21 pieces of data for each individual product are entered into the GUDID for the USA and into the European Database on Medical Devices (EUDAMED). In all of these respects, rantronik uses its relevant expertise to advise companies from across the medical engineering and pharmaceuticals industries.

Experience our expertise for yourself
Do you have any questions, or want to find out more?
Then please get in touch by telephone, e-mail or using our contact form – we look forward to hearing from you.